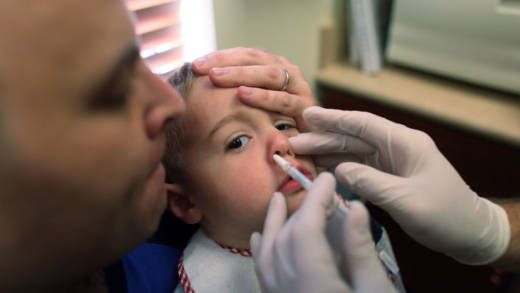
The CDC recommends everyone over the age of 6 months be vaccinated every year against the flu, unless there is a medical reason to avoid the vaccine.
Yet only about 60 percent of children between the ages of 6 months and 17 years got vaccinated against flu in 2016-2017, the most recent year for which data are available. Roughly eight out of every 10 children who died from flu weren’t vaccinated, said Dr. Lisa Grohskopf, a medical officer in the CDC’s influenza division.
The differing advice from the CDC and the AAP on AstraZeneca’s FluMist could befuddle parents and pediatricians.
“There’s no question that ideally we would like for the CDC and the AAP to be completely harmonized” when it comes to recommendations, said Bernstein who is also a member of the Advisory Committee on Immunization Practices, which guides the CDC on vaccine decisions.
“Both groups are harmonized in wanting as many children to receive flu vaccine as possible each and every year,” said Bernstein. “When recommendations are not perfectly harmonized, it does pose the possibility for confusion.”
But any confusion might be mitigated by the fact that finding FluMist might not be easy this flu season.
The decision to once again recommend it — effectively giving doctors and pharmacists a go-head to use it again — was made by the ACIP in late February. By that point, though, many flu vaccine orders would already have been placed for the 2018-2019 season.
While dozens of lots of vaccine products made by Sanofi Pasteur, GlaxoSmithKline, and Seqirus have been given the green light for distribution by the Food and Drug Administration, no lots of FluMist had cleared that hurdle as of Aug. 30.
It has been a rocky few years for FluMist, which once was deemed more effective in children than injectable flu vaccine. But just after ACIP gave FluMist a rare preferential recommendation in 2014, performance problems came into view. By the 2016-2017 flu season, the CDC’s vaccine advisers recommended it not be used in the U.S. ACIP retained that position for the following flu season as well.
The problem was vaccine effectiveness studies that are done every year showed FluMist had not been offering much if any protection against the influenza A virus family H1N1.
It wasn’t clear why the vaccine’s performance was so poor in the U.S. during the 2013-2014 and 2015-2016 seasons. To make matters more confounding, other countries that use FluMist — Canada, Finland, and Britain among them — did not see the puzzling lack of effectiveness.
Unlike injectable flu vaccine, FluMist contains live viruses. The viruses in the vaccine, which is puffed up a nostril of the recipient, initiate the infection process, thereby activating an immune response. But the viruses in the vaccine are weakened and don’t induce illness.
In response to its U.S. performance problems, AstraZeneca reformulated the H1N1 portion of the vaccine. There is some evidence that suggests the updated vaccine may be more effective — although the company hasn’t been able to do studies in children to confirm that. H3N2 viruses have dominated in the last two flu seasons here.
“They provided some evidence that appeared promising that the new virus that’s going to go into the vaccine this season in the U.S. is a bit more fit,” said Grohskopf. “It is promising evidence that what is at least believed to be the root cause of the problem has been fixed.”
Bernstein said the AAP was not as convinced by the data as the CDC. He voted against recommending FluMist for the 2018-2019 season at the February ACIP meeting.
Another place the CDC and AAP advice diverges slightly relates to when children should be vaccinated.
The AAP recommendations, published Monday in the journal Pediatrics, suggest pediatricians should start urging parents to vaccinate their children as soon as flu vaccine becomes available. That can be as early as late July or August, which — depending on when flu activity picks up — is often months ahead of when children will face a flu threat. Flu season often peaks in January or February, and influenza B viruses, which are particularly hard on children, often circulate late in the winter.
There are some studies that have shown protection from flu vaccine starts to wear off as the season wears on.
The CDC’s recommendations, published Aug. 24, suggested people should be vaccinated by the end of October, when flu activity can start to tick up.
Grohskopf acknowledged the variability and unpredictability of influenza makes it tough to know when to advise people to get the vaccine. “What we can say is the best time to get vaccinated is probably a couple of weeks before flu starts circulating where you are. But we can never really tell people when that is,” she admitted.
One thing is clear though, she said. Parents of young children should start the vaccination process earlier. That’s because children under 8 years old who haven’t had at least two flu vaccines in their life need two doses of vaccine given at least four weeks apart. The second shot should be by the end of October, the CDC guidance said.