The Food and Drug Administration on Wednesday approved a futuristic new approach to treating cancer, clearing a Novartis therapy that has produced unprecedented results in patients with a rare and deadly cancer. The price tag: $475,000 for a course of treatment.
That sounds staggering to many patients — but it’s far less than analysts expected.
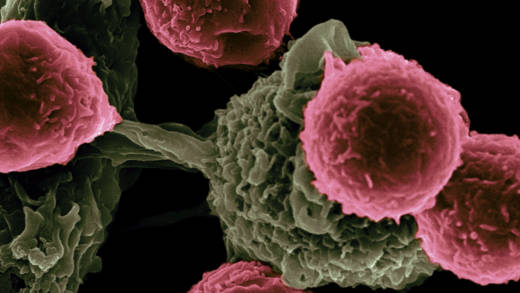
The therapy, called a CAR-T, is made by harvesting patients’ white blood cells and rewiring them to home in on tumors. Novartis’s product is the first CAR-T therapy to come before the FDA, leading a pack of novel treatments that promise to change the standard of care for certain aggressive blood cancers.
Novartis’s therapy is approved to treat children and young adults with relapsed acute lymphoblastic leukemia. It will be marketed as Kymriah.
The treatment’s approval has looked a foregone conclusion for months, but its potential price has been the subject of speculation and debate. Novartis picked the $475,000 price tag in an effort to balance patient access to Kymriah while giving the company a return on its investment, said Bruno Strigini, Novartis’s head of oncology, in a conference call Wednesday. The cost is below Wall Street analyst expectations, which reached as high as $750,000 for a dose. And it’s considerably cheaper than the roughly $700,000 price tag that U.K. regulators said would be fair considering Kymriah’s potential benefits.