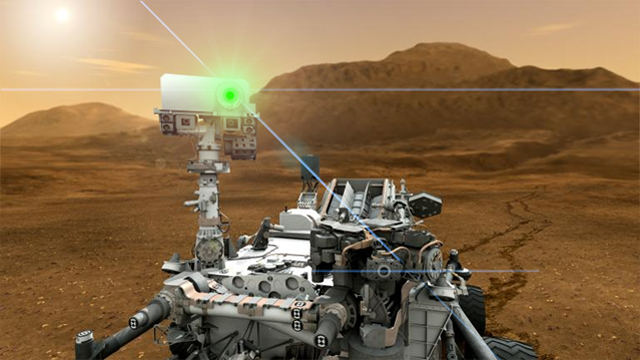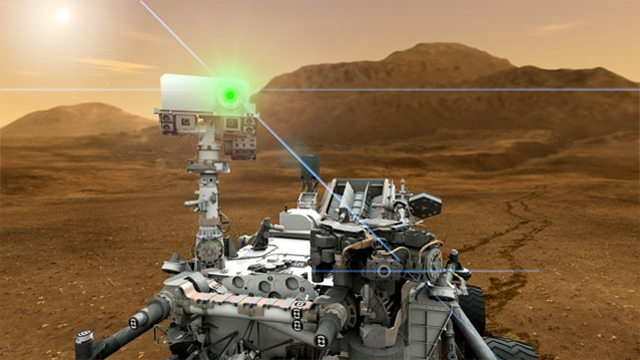
Space exploration has caught up with science fiction (again): we have deployed laser-armed nuclear-powered robot on Mars, and nearly two weeks after landing, NASA's Mars Science Laboratory, the rover Curiosity, has fired that weapon on a Martian...rock.
Certain images from sci-fi classics come to mind. Remember, "The Day the Earth Stood Still" when Gort, the golem-like space-cop-bot, went about melting soldiers' rifles with an incinerating ray? Recall how the Robinsons became "lost in space" when their reprogrammed automaton when berserk with its electric bolts? And who can forget when dear old R2D2 finally let slip that he/she/it is armed and dangerous when he/she/it zapped the little gremlin from the planet FrankOz? This is the droid you want...Ah, good times.
The rover Curiosity is equipped with ten different scientific experiments, perhaps the flashiest one being a spectrometer. A spectrometer sorts and analyzes the wavelengths (colors) of light emitted by an object: the light's spectrum. Every chemical element and compound shines a unique spectrum, so sorting out the different wavelengths present reveals the source's composition.
Spectrometers are a powerful tool for astronomers and geologists alike. We first learned that stars are composed mostly of hydrogen and helium through spectroscopic measurements, and in the same way we also detected the presence of dozens of other chemicals on the sun. (As an aside, helium was named for the sun—Helios, in Greek—because it was detected there by spectrometers before it was discovered on Earth.)
But in an environment like Mars, where the visible light shining from the rocks and soil of the landscape is merely reflected sunlight, using a spectrometer doesn't tell you much about the composition of the rocks and soil! That's where the dangerous, high-powered laser comes in. By firing the laser on a rock or a spot of soil, a small bit of it is vaporized and glows with its own light. Then, Curiosity's spectrometer can do its work and analyze the emitted light.