“The cells understand they've been attacked and they commit suicide,” said Gilad Doitsh, a research scientist and the lead author of the study, which was published last Wednesday in the scientific journal Cell.
“It’s as though the virus needs to go through several rooms of the cell without activating the alarm,” he said, “and if it stalls in one of those rooms, the alarm sounds and the cell kills itself.”
 Gilad Doitsh, a molecular biologist at the Gladstone Institute of Virology and Immunology in San Francisco, studies the HIV virus. (Credit: Chris Goodfellow, Gladstone Institutes)
Gilad Doitsh, a molecular biologist at the Gladstone Institute of Virology and Immunology in San Francisco, studies the HIV virus. (Credit: Chris Goodfellow, Gladstone Institutes)
Doitsh and his colleagues, working at the University of California-San Francisco’s Mission Bay campus, focused their research on a type of white blood cells called 'CD4 T cells'. The cells play a critical role in fighting infection from bacteria and viruses.
CD4 T cells are also known as ‘helper cells’ because they help the production of a specific immune response – antibodies – to a specific bacteria or virus. After a bacteria or virus enters the body, communication occurs between different immune cells in the lymph tissues to develop an antibody which the immune system will then remember and reproduce if it encounters the same type of bacteria or virus weeks, months or even years later.
Though the human body has 10 types of immune cells, the main target of HIV is CD4 T cells. But despite this limited target, the immune system still can’t produce an antibody to marshal an effective, one-size-fits-all attack against the virus.
"The major challenge of HIV is its ability to mutate or change. So we're constantly chasing the virus,” said Dr. Warner Greene, director of the Gladstone Institute of Virology and Immunology and a senior author of the study. "It requires combination antiretroviral therapy to attack it. Single drugs will not work,” he added.
At least 26 drugs have been approved by the FDA to treat HIV, which infects roughly 60,000 people in the U.S. each year. Worldwide, more than 33 million people are living with HIV, with 10 million of them awaiting drug treatment, according to the United Nations.
Doitsh’s team takes an unusually thorough approach when investigating the spread of HIV in the human body.
“Our lab is very, very unique. We process human tissues from the tonsils, spleen, cervix, skin, colon, blood, semen - any tissue where HIV multiplies or that serves as a vehicle to transmit HIV from person to person,” he said. “You see the virus behave differently depending on the tissue it has infected.”
According to Doitsh, most other labs researching HIV and AIDS study the virus in blood because of the ease of finding copies of the virus there, even though it’s not the site where the virus actually replicates, or makes copies, of itself. So Doitsh used tonsil and spleen tissues because they’re the principal places where the virus replicates. Being lymph organs, the tonsil and spleen also contain large amounts of CD4 T cells.
The researchers exposed the tonsil and spleen tissues to HIV. Then, to tell when the virus was actively replicating in the CD4 T cells, the scientists used a marker which turned green when the virus had fully hijacked the genetic machinery of the cell, tricking the cell into making thousands of new copies of the virus.
After binding to the CD4 T cell, the virus can’t directly enter the cell’s nucleus to take over its DNA, the genetic program which runs the cell. And since the virus doesn’t have DNA of its own, it has to convert its RNA, the genetic program on which it runs, to DNA in a process called ‘reverse transcription’.
When it has converted its RNA to DNA, HIV can then speak the genetic language of the CD4 T cell. It enters the infected cell’s nucleus and boots up its genetic program, which instructs the cell to make new copies of the virus, thereby amplifying HIV in the body.
And although all the CD4 T cells eventually died from infection by the virus, how they died was a bit of a surprise.
Only about 5 percent of the cells that died were "productively", or fully, infected with HIV. The other 95 percent “bystander” cells died not from the virus itself but from an alarm signal inside the cell which triggered it to commit suicide after it was exposed to the virus but before the virus could replicate inside it.
“These CD4 T cells commit suicide to protect the body, but you can't have all the soldiers commit suicide because then the country has no more army,” said Doitsh.
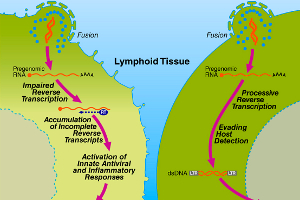 Graphic showing to the left, a CD4 T cell that commits suicide after infection by HIV, whereas the illustration on the right depicts a cell that allows HIV to replicate in it. (Click on image for a larger version) (Credit: Gilad Doitsh, Gladstone Institute of Virology and Immunology)
Graphic showing to the left, a CD4 T cell that commits suicide after infection by HIV, whereas the illustration on the right depicts a cell that allows HIV to replicate in it. (Click on image for a larger version) (Credit: Gilad Doitsh, Gladstone Institute of Virology and Immunology)
To figure out exactly when these CD4 T cell ‘soldiers’ committed suicide, Doitsh exposed the CD4 T cells to different antiretroviral drugs like AZT, AMD3100 and T20, which attack the virus at various stages of its life cycle.
While AZT failed to rescue the infected CD4 T cells from dying, the other drugs were able to save the CD4 T cells because they attacked the virus before it entered the nucleus of the cell. But Doitsh and his colleagues didn’t know if the cells were committing suicide early on, when the virus merely touched the cell to enter it, or later, when the virus had already entered the cell.
So they used another drug, Efavirenz, which attacks HIV after it has entered the cell but before it starts making copies of itself from within the cell’s nucleus. When the CD4 T cells were exposed to Efavirenz, they survived.
“People thought that the virus could be on the outside of the cell, that it’s enough to put the key into the lock to signal the destruction of the room,” said Doitsh. “We showed that it’s not the key in the lock but that the virus has to actually go inside the room - or the cell - for the cell to die.”
“It hasn't been very clear why these so-called ‘bystander cells’ also die,” said Tae-Wook Chun, a staff scientist who has been studying HIV for 12 years at the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland. “The researchers found that these cells also get infected, although HIV doesn’t complete its life cycle in these cells. So they solved one of the mysteries in HIV research,” he added.
But that’s not all they found.
“The second huge surprise we found was that the mechanism of cell death was not a silent one. These cells do not go silently into the night,” said Greene.
Instead, Greene said they died a “fiery death,” causing “significant inflammation” as they erupted their cellular contents and released a chemical signal which recruited healthy CD4 T cells to the site of infection.
“It’s like a 911 call, with the dying cell saying, ‘we need more white blood cells to fight whatever infection is going on’” said Doitsh. “The new CD4 T cells want to help but HIV is there,” he added.
“It’s a vicious cycle, whereby the dying CD4 T cells release inflammatory signals that attract more cells to die,” said Greene.
This inflammatory response the researchers observed offers an interesting avenue to explore for the possible development of more effective HIV drugs.
“I think there are possibilities that we could block the inflammation component and therefore greatly modify the progression of disease,” said Greene. He also pointed out that primates like chimpanzees who are infected with a strain of HIV experience a drop in CD4 T cells but they don’t show signs of inflammation, nor crucially, do they develop AIDS.
Tae-Wook Chun at NIAID, however, cautioned that additional studies need to be done, perhaps with tissue from already infected HIV patients, to determine the clinical relevance of the findings.
The scientists will next try to track down the cellular sensor which detects HIV in the cell body and triggers the cellular suicide. They also want to study the virus to see if it has genes which allow it to turn off the cellular sensor in that small percentage of cells which become a host for making additional copies of the virus.
 HIV researcher Mauricio Montano at work at the Gladstone Institute of Virology and Immunology. (Credit: Chris Goodfellow, Gladstone Institutes)
HIV researcher Mauricio Montano at work at the Gladstone Institute of Virology and Immunology. (Credit: Chris Goodfellow, Gladstone Institutes)
Gilad Doitsh spent seven years doing the research in Greene’s lab and confirming his results before he was confident enough to present his findings for publication. Today, he advises three doctoral students who are beginning to investigate the behavior of HIV, much like he did nearly a decade ago.
“I tell them we are like detectives,” said Doitsh. “We have a suspect – HIV. We’re sure he committed murder but we have to collect the best evidence to convince the jury so that this guy can go to jail.”
“You have to know every aspect of HIV. You take baby steps but if every step is solid, then you have enough proof to convict the suspect,” he added.
 HIV researcher Gilad Doitsh talks with Dr. Warner Greene (seated) at the Gladstone Institute of Virology and Immunology in San Francisco. (Credit: Chris Goodfellow, Gladstone Institutes)
HIV researcher Gilad Doitsh talks with Dr. Warner Greene (seated) at the Gladstone Institute of Virology and Immunology in San Francisco. (Credit: Chris Goodfellow, Gladstone Institutes) 


